Current Issue
The Triglyceride-glucose (TyG) Index in the Association of Non-alcoholic Fatty Liver Disease, Obesity and Insulin Resistance
Mohamed Ridha GUEDJATI*, Adeila Dallel TAIBI, Hafsa Bougroura
Service de Physiologie Clinique et Explorations Fonctionnelles Métaboliques et Nutrition du CHU de Batna, Centre Hospitalier Universitaire Benflis Touhami de Batna. Allées Mohamed Boudiaf Batna, Algérie
*Corresponding Author: Mohamed Ridha GUEDJATI, Service de Physiologie Clinique et Explorations Fonctionnelles Métaboliques et Nutrition du CHU de Batna, Algérie; Email: [email protected]
Received Date: February 26, 2024
Publication Date: March 20, 2024
Citation: GUEDJATI MR, et al. (2024). The Triglyceride-glucose (TyG) Index in the Association of Non-alcoholic Fatty Liver Disease, Obesity and Insulin Resistance. Diab Res. 1(1):1.
Copyright: GUEDJATI MR, et al. © (2024).
ABSTRACT
Aims: Non-alcoholic fatty liver disease is strongly linked to obesity and insulin resistance. The triglyceride-glucose index (TyG index) has been proposed as a reliable biomarker of insulin resistance. Our aims is to study the contribution of the TyG index as a marker in the association of non-alcoholic fatty liver disease, obesity and insulin resistance. Material and method: This was a cross-sectional study of obese women (age>18years, BMI≥29.9Kg/m2). Non-alcoholic hepatitis steatosis was confirmed by abdominal ultrasound. The viral origin of the liver disease was eliminated by microbiological test. A lipid profile was performed. The TyG index was calculated according to the formula Ln[fasting triglycerides(mg/dL)×fasting glucose(mg/dL)/2. The relations between TyG index body composition and lipid profile was measured. Results: 42 obese women participated in our work. Age=50.80±10.33 years. BMI=40.80±5.09 Kg/m2; weight=98.1±15.99 Kg. Fasting blood sugar was 1.18±0.34 g/L. Triglycerides were 1.37±0.47 g/L. Our study demonstrated that 83% (n= 35) have, in addition to non-alcoholic fatty liver disease, a high TyG index (4.75+/-0.25) in favour of insulin resistance. The TyG index is strongly linked to fasting glucose (rs=0.83) and fasting triglycerides (rs=0.78). A statistically significant link was found between the TyG index and age (τ = 0.21, p=0.04), the TyG index and total cholesterol (τ = 0.33, p=0.001). Conclusions: The TyG index is a topical biomarker. This clue is practical. It appears to have a place in the association, obesity, non-alcoholic fatty liver disease and insulin resistance.
Keywords: TyG index, insulin resistance, obesity, non-alcoholic fatty liver disease
INTRODUCTION
Obesity is a global health problem. It is estimated that around 1.5 billion adults worldwide are overweight, of whom around 200 million men and 300 million women are obese [1]. According to the WHO, overweight and obesity are defined as an abnormal or excessive accumulation of fat that is harmful to health. A person is considered overweight when their body mass index (BMI) is greater than 25kg/m2 and obese when it is greater than 30kg/m2. Obesity leads to the development of a number of co-morbidities, including type 2 diabetes (T2DM), non-alcoholic fatty liver disease (NASH), hypertension, hyperlipidaemia, chronic kidney disease, cardiovascular disease (CVD), obstructive sleep apnea, osteoarthritis and malignant tumors, resulting in increased mortality in obese people [2]. The increase in the prevalence and severity of NASH is linked to rising levels of obesity [3]. NASH has now become one of the leading causes of chronic liver disease. The prevalence of obesity-induced NASH and the resulting morbidity may be considered the major health crises of the next decade in the industrialized world [4,5]. Similarly, mortality from NASH continues to rise, while mortality from viral hepatitis is declining [6]. Non-alcoholic fatty liver is the most common liver disease in Western countries. The global prevalence of NASH is estimated to be around 25% and is expected to increase to 33.5% by 2030 [7]. Non-alcoholic fatty liver disease (NASH) is characterized by the diffuse accumulation of triglycerides in hepatocytes. This steatosis is not caused by excessive alcohol consumption or other causes that affect the liver [8]. NASH is generally attributed to obesity-induced insulin resistance [8]. NASH can be complicated by cirrhosis or even hepatocellular carcinoma [7]. NASH is also considered a risk factor for extra-hepatic diseases such as cardiovascular disease (CVD), chronic kidney disease, colorectal cancer, type 2 diabetes mellitus (T2DM) and osteoporosis [7]. There are currently no approved pharmacological treatments for NASH, with the exception of lifestyle changes [9]. Early detection of patients at risk of NASH using simple and effective diagnostic methods is crucial.
Early identification of people at high risk of NASH enables preventive strategies to be put in place and slows down the morbidity and mortality of liver-related diseases. Traditionally, the diagnosis of NASH has required various techniques such as liver ultrasound, magnetic resonance imaging and biopsy [10]; however, some of these techniques are either invasive or expensive and have limited applicability in clinical practice. The most widely used diagnostic tool is liver ultrasound. NASH is often associated with multiple metabolic disorders that may be partly explained by insulin resistance. The triglyceride-glucose index (TyG) is derived from the formula:
[ln(fasting triglycerides (mg/dL) * fasting blood glucose (mg/dL)/2] [11], is increasingly used because of its better performance in estimating insulin resistance. In practice, insulin resistance is measured by the homeostasis model assessment (HOMA) [12-14]. It is derived from the formula [Fasting blood glucose (mmol/L) * Fasting blood insulin (mui/mL)/22.5]. It is used to assess insulin resistance (IR). The HOMA-IR index requires a delicate biochemical technique [15]. Given its ease of acquisition and calculation, the TyG index is widely accepted and used in clinical practice to assess IR [14,16]. It uses two serum parameters, triglycerides and glucose, which are strongly linked to the regulation of insulin secretion. Insulin sensitivity can be subtly affected by the metabolism of these two parameters.
Insulin resistance, which increases lipolysis [17] and stimulates de novo lipogenesis favouring the production and storage of triglycerides in the liver [18] as well as fat reserves. These reserves are directly associated with liver inflammation [19] and play an important role in the pathogenesis of NASH. Triglyceride accumulation in hepatocytes is due to an imbalance between lipid accumulation and elimination [17]. Several causes can lead to this excessive accumulation of triglycerides, such as increased fat intake due to a high-fat diet, reduced metabolism of fats in the form of very low density lipoproteins (VLDL) and triglycerides, reduced beta oxidation of free fatty acids and de novo lipogenesis [18]. This excess of triglycerides leads to the development of NAFLD (Non Alcoholic Fatty Liver Disease) through liver dysfunction [17,18]. Furthermore, the accumulation of fatty liver is linked to insulin resistance [19]. Excess fatty acids from lipogenesis and fatty acid synthesis accumulate in peripheral tissues, the liver and adipose tissue, leading to peripheral insulin resistance [20]. All these mechanisms contribute to the metabolic disorders that may characterise NASH, which is an inflammatory component of NAFLD [21]. Non-alcoholic fatty liver disease (NASH) is strongly linked to obesity, and insulin resistance is the key pathogenic factor in the development of NASH. The TyG index is dependent on disorders affecting glucose and triglyceride metabolism. It is possible that it could provide information on insulin resistance in obese people with non-alcoholic fatty liver disease.
OBJECTIVE
The aim of this study was to investigate the contribution of the TyG index as a metabolic marker in obese women with hepatic steatosis in favor of non-alcoholic fatty liver disease.
METHOD AND MATERIALS
This is a descriptive cross-sectional study carried out between January and December 2022 in the metabolic physiology and nutrition department of Batna University Hospital. The inclusion criteria were met by 42 women (aged 18 years and over, female, obese BMI≥29.9 kg/m2 and having undergone a liver ultrasound scan which revealed steatosis). Children, men, people without abdominal ultrasound results, pregnant women and those with cancer or viral hepatitis were excluded from this study. Also we excluded people with diabetes and those presenting heart disease. Weight and height were measured in patients wearing light clothing and no shoes.
Body composition was analysed using 8-electrode impedancemetry of the Tanita® BC 418 MA type. A fasting blood glucose level and a lipid profile were determined. Patients were classified according to their degree of obesity on the basis of their BMI. The cardiometabolic profile was identified on the basis of fasting blood glucose and lipid profiles. The TyG index was calculated using the formula TyG index=ln[fasting triglycerides (mg/dl)X fasting blood glucose (mg/dl)]/2 [22].
Statistical values are expressed as percentages, mean plus or minus standard deviation. Spearman's and Kendall's tests were used to look for associations between the TyG index, body composition and lipid balance. Statistical analysis was performed using the biosta TGV website [23]. A p value of <0.05 was considered statistically significant.
RESULTS
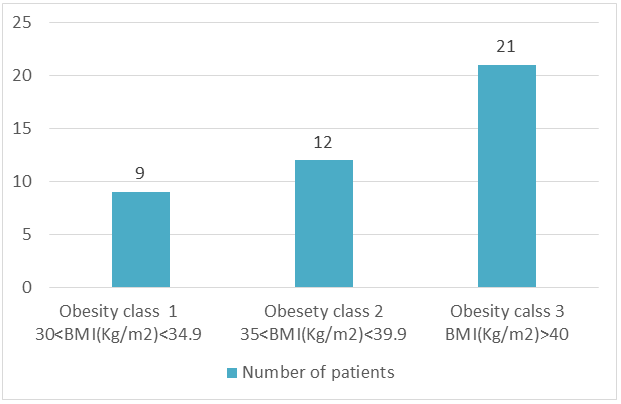
42 obese women took part in our study (Table 1). The mean age was 50.80±10.33 years. The mean weight was 98.1±15.99 kg and the mean BMI was 40.80±5.09 kg/m2. Half (n=21) of our patients were morbidly obese with a BMI ≥ 40 Kg/m2 (Figure 1).
Figure 1: Distribution according to stages of obesity.
Table 1: General data of the study population.
|
|
Means |
Standard deviation (+/-) |
|
Age (years) |
50.80 |
10.33 |
|
Weight (Kg) |
98.1 |
15.99 |
|
BMI (kg/m2) |
40.80 |
5.09 |
|
Fasting blood glucose (g/L) |
1.18 |
0.34 |
|
TG (g/L) |
1.37 |
0.47 |
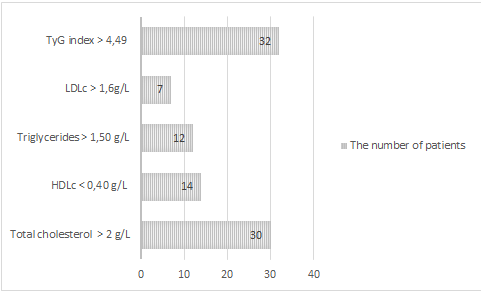
In addition to hepatic steatosis, 80% (n=32) of our patients had a TyG index > 4.49 in favour of insulin resistance. 71% (n=30) had a cholesterol level > and almost (1/3) had either a triglyceridaemia > 1.5 g/L or a low HDLc (Figure 2).
Figure 2: The number of patients with metabolic disorders.
In the 35 obese women with non-alcoholic fatty liver disease, the TyG index was (4.75+/-0.25) in favour of insulin resistance. The TyG index was strongly associated with glycaemia (rs=0.83) and triglycerides (rs=0.78), but this association was not statistically significant (table 2). However, a statistically significant link was found between TyG index and age (τ=0.21, p=0.04), TyG index and total cholesterol (τ =0.33, p=0.001).
Table 2: The different correlations of the TyG index with the different study parameters.
|
|
rs |
P value* |
τ |
P value* |
|
Weight(KG) |
-0,08 |
0,57 |
-0,06 |
0,52 |
|
BMI (Kg/m2) |
-0,01 |
0,93 |
-0,04 |
0,96 |
|
Age (Years) |
0,29 |
0,05 |
0,21 |
0,04 |
|
Triglycerides (g/L) |
0,83 |
NS |
0,69 |
NS |
|
Fasting blood glucose (g/L) |
0,78 |
NS |
0,59 |
NS |
|
Total cholesterol (g/L) |
0,48 |
0,001 |
0,33 |
0,001 |
|
Total body fat (g/L) |
-0,10 |
0,51 |
-0,07 |
0,51 |
|
Visceral fat |
-0,40 |
0,77 |
-0,03 |
0,77 |
In 35 obese women with non-alcoholic fatty liver disease, the TyG index was (4.75+/-0.25) in favour of insulin resistance. The TyG index was strongly associated with glycaemia (rs=0.83) and triglycerides (rs=0.78), but this association was not statistically significant. However, a statistically significant link was found between TyG index and age (τ=0.21, p=0.04), TyG index and total cholesterol (τ =0.33, p=0.001).
DISCUSSION
Non-alcoholic fatty liver (NASH) is characterised by the diffuse accumulation of triglycerides in the hepatocytes. This accumulation is not caused by excessive alcohol consumption or other causes of liver disease [8]. NASH is generally attributed to obesity-induced insulin resistance [8]. It is considered a risk factor for extra-hepatic diseases such as cardiovascular disease (CVD), chronic kidney disease (CKD), colorectal cancer, type 2 diabetes mellitus (T2DM) and osteoporosis [7]. The aim of this study was to investigate the contribution of the TyG index as a cardiometabolic biomarker in obese women with non-alcoholic fatty liver disease confirmed by ultrasound imaging. We found a TyG index > 4.49 in 32 people (N = 42). Sonia Hossain's study in 2020 [20] showed that the onset of hepatic steatosis increased significantly with increasing levels of TyG index. Our results are consistent with the cohort conducted by Kitae et al [24]. In this study the authors showed that the TyG index is significantly associated with the incidence of NASH based on ultrasound in the general adult population. NASH is becoming a silent global epidemic with a significant health and economic burden [25]. NASH is largely ignored by patients because there are no obvious clinical signs. However, if left untreated, NASH can lead to serious complications such as cirrhosis and even hepatocellular carcinoma [26]. Therefore, early detection and intervention for NASH patients is essential to prevent further complications of NASH and other associated chronic diseases such as diabetes. IR is the major contributor to the pathogenesis of NASH via increased supply of free fatty acids to the liver, inadequate fatty acid oxidation and increased de novo lipogenesis [26]. IR is evidenced by the HOMA-IR (Homeostasis Model Assessment - Insulin Resistance) test, which should measure serum insulin levels; however, widespread screening in primary care settings using HOMA-IR does not appear to be evident [27]. Similar to our findings, a cohort study by Rivière et al [25], used liver biopsy to detect NASH in obese patients. This study showed a strong association between the TyG index and NASH. The TyG index is a convenient new surrogate marker for RI that has recently gained ground due to its simplicity of calculation. In addition, the TyG index shows some predictive superiority of IR over HOMA-IR [28]. In addition, the TyG index has been shown to be the best test for detecting simple steatosis compared with other NASH indices, such as SteatoTest, NashTest and the hepatic steatosis index [29]. These indices are not widely used in clinical practice, due to their complexity and difficulty of calculation, as well as their high cost [29]. The TyG index is easy to calculate and can therefore be a practical tool for screening for NASH. This index even surpasses the HOMA-IR in the prediction of NASH [30]. Previous studies have demonstrated a strong dose-response association between TyG index and NASH when categorizing TyG into quartiles [31- 34]. Guo et al [33] showed that the prevalence of NASH increased from 30.9% to 53.3%, to 71.7%, to 86.4% in increasing TyG quartiles (Q1, Q2, Q3 and Q4, respectively; p-value for trend < 0.001). Huanan et al [32] further demonstrated that the higher the level of the TyG index, the higher the incidence of NASH, whether the TyG index was analysed as a continuous or categorical variable. However, Khamseh et al suggest that the TyG index combined with other indices such as obesity stage and waist circumference may be more accurate than the TyG index alone [35]. In our work, we found no link between BMI and TyG index. This was a single-centre study with a relatively small sample size. Evidence of liver damage was based on ultrasound findings, which reported radiological lesions in favour of hepatic steatosis. Several aspects need to be clarified. The definition of steatotic liver disease was re-examined by a group of international experts in 2020 [36,37]. The group reached a consensus in favour of changing the nomenclature. A distinction has already been made between non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MAFLD) [36]. According to these authors, MAFLD is defined by the presence of hepatic steatosis, in addition to one of the following three criteria: overweight or obesity, the presence of T2DM or the presence of metabolic dysfunction. We believe that our population precisely meets the definition of MAFLD (metabolic dysfunction-associated fatty liver disease) rather than NASH. These are obese women with fatty liver disease, half of whom (n=21) are morbidly obese. Histologically, NAFLD is a common term that encompasses a broad spectrum of diseases ranging from isolated steatosis known as simple hepatic steatosis or Non Alcoholic Fatty Liver (NAFL) to NASH, the latter potentially leading to hepatic fibrosis, cirrhosis or hepatocellular carcinoma [38,39]. At the cellular level, in addition to hepatic steatosis, NASH is defined by lobular inflammation and signs of hepatocyte damage (distinguished by ballooning of the hepatocytes) with varyin degrees of fibrosis [40]. The cohort study conducted by Rivière et al [25] used liver biopsy in obese patients to detect NASH. A strong association was demonstrated between the TyG index and NASH. NASH is a silent global epidemic with a significant health and economic burden [24]. It is a silent disease that may be underpinned by several pathophysiological mechanisms. Fat accumulation in the visceral liver is linked to insulin resistance [18]. Excess fatty acids from lipogenesis and triglyceride synthesis from excess glucose accumulate in peripheral tissues, adipose tissue and the liver, promoting peripheral insulin resistance [19]. Our work focuses on the MAFLD entity [41] where multiple metabolic disorders have been identified, hypercholesterolemia, hypertriglyceridemia, a glycaemic disorder favouring pre-diabetes and an increased risk of cardiovascular disease (low HDLc and/or high LDLc). These results suggest that the syndromic grouping of metabolic disorders in an obese person with radiological signs of hepatic steatosis exposes them to major risks of cardio-metabolic damage. Similarly, the presence of obesity and hyperlipidaemia is also associated with an increased risk of progressive liver disease [42]. Visceral fat appears to be the predominant factor in the genesis of insulin resistance. We found a negative correlation between visceral fat and the TyG index, which seems contradictory. Metabolic syndrome is based on the combination of at least two or three criteria, including the location of abdominal fat. Measurement of waist circumference confirms this location and assesses its extent [43]. According to the guidelines of certain learned societies [39], all people with incidental steatosis should be screened for metabolic syndrome, independently of functional liver tests. Although an increase in waist circumference was associated with a higher risk of NAFLD, independently of abdominal obesity status [44], we support the hypothesis that waist circumference, which is a simple and practical anthropometric tool, is not in itself sufficient to judge the metabolic severity of visceral fat. Physiopathological mechanisms, involving the nature of the fatty tissue and above all the secretion of mediators by the latter, may explain the appearance (or not) of a metabolic disorder such as insulin resistance.
As demonstrated in this study, the TyG index can consolidate the existence of a Mets-MAFLD syndromic cluster. However, it cannot, on its own, be a predictor of this disorder. Parameters such as TyG-BMI or TyG-WC appear to be more relevant [8]. They should therefore be promoted.
CONCLUSION
Obesity aggravates non-alcoholic liver diseases, NAFL, MAFLD, NAFLD and NASH. Early diagnosis of these diseases enables complications to be prevented more effectively. Diagnosis is based on radiological and biological techniques, including HOMA-IR. The TyG index is a topical biomarker and appears to be a simple, practical and affordable tool for screening for insulin resistance.
REFERENCES
- Polyzos SA, Kountouras J, Mantzoros CS. (2019). Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 92:82–97.
- Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. (2016). Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 65(8):1017–1025.
- Eslam M, Valenti L, Romeo S. (2018). Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 68(2):268–279.
- Polyzos SA, Kountouras J, Mantzoros CS. (2016). Adipokines in nonalcoholic fatty liver disease. Metabolism. 65(8):1062–1079.
- Polyzos SA, Mantzoros CS. (2015). Leptin in health and disease: facts and expectations at its twentieth anniversary. Metabolism. 64(1):5–12.
- Kumar R, Priyadarshi RN, Anand U. (2019). Non-alcoholic fatty liver disease: Growing burden, adverse outcomes and associations. J Clin Transl Hepatol. X(X):1–11.
- Song S, Son D H, Baik S J, Cho W J, Lee Y J. (2022). Triglyceride glucose-waist circumference (TyG-WC) is a reliable marker to predict non-alcoholic fatty liver disease. Biomedicines. 10(9):2251.
- Kwak M-S, Kim D. (2018). Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 33(1):64–74.
- Rinella ME. (2015). Nonalcoholic fatty liver disease: A systematic review. JAMA. 313(22):2263.
- Leung PB, Davis AM, Kumar S. (2023). Diagnosis and management of nonalcoholic fatty liver disease. JAMA. 330(17):1687.
- Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Dulbecco CA, Reaven GM. (2017). Comparison of two surrogate estimates of insulin resistance to predict cardiovascular disease in apparently healthy individuals. Nutr Metab Cardiovasc Dis. 27(4):366–373.
- Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, et al. (2016). Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 47(5):382–387.
- Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. (2022). Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 32(3):596–604.
- Toro-Huamanchumo CJ, Urrunaga-Pastor D, Guarnizo-Poma M, Lazaro-Alcantara H, Paico-Palacios S, Pantoja-Torres B, et al. (2019). Triglycerides and glucose index as an insulin resistance marker in a sample of healthy adults. Diabetes Metab Syndr. 13(1):272–277.
- Reaven G. (2011). Wanted!: A standardized measurement of plasma insulin concentration. Arterioscler Thromb Vasc Biol. 31(5):954–955.
- Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. (2020). Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int J Endocrinol. 2020:1–7.
- Kawano Y, Cohen DE. (2013). Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 48(4):434–441.
- Yu J, Marsh S, Hu J, Feng W, Wu C. (2016). The pathogenesis of nonalcoholic fatty liver disease: Interplay between diet, gut Microbiota, and genetic background. Gastroenterol Res Pract. 2016:1–13.
- Edmison J, McCullough AJ. (2007). Pathogenesis of non-alcoholic steatohepatitis: Human data. Clin Liver Dis. 11(1):75–104.
- Hossain S, Sultana S, Zaman KMS, Shafiq S, Rahman AKMS, Hossain SMZ, et al. (2020). Triglyceride and glucose index (TyG) is a reliable biomarker to predict non-alcoholic fatty liver disease. J Biosci Med (Irvine). 08(11):124–136.
- EASL, EASD, EASO. (2016). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 64(6):1388–1402.
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. (2008). The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 6(4):299-304.
- BiostaTGV - Statistiques en ligne. Sentiweb.fr.
- Kitae A, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. (2019). The triglyceride and glucose index is a predictor of incident nonalcoholic fatty liver disease: A population-based cohort study. Can J Gastroenterol Hepatol. 2019:1–7.
- Rivière B, Jaussent A, Macioce V, Faure S, Builles N, Lefebvre P, et al. (2022). The triglycerides and glucose (TyG) index: A new marker associated with nonalcoholic steatohepatitis (NASH) in obese patients. Diabetes Metab. 48(4):101345.
- Lazarus JV, Colombo M, Cortez-Pinto H, Huang TT-K, Miller V, Ninburg M, et al. (2020). NAFLD — sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol. 17(7):377–379.
- Johnston MP, Patel J, Byrne CD. (2020). Causes of mortality in non-alcoholic fatty liver disease (NAFLD) and alcohol related fatty liver disease (AFLD). Curr Pharm Des. 26(10):1079–1092.
- Utzschneider KM, Kahn SE. (2006). The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 91(12):4753–4761.
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. (2000). Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 23(1):57–63.
- Simental-Mendía L E, Simental-Mendía E, Rodríguez-Hernández H, Rodríguez-Morán M, Guerrero-Romero F. (). The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann Hepatol. 15(5):715–720.
- Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, et al. (2019). Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul). 34(2):179.
- Huanan C, Sangsang L, Amoah AN, Yacong B, Xuejiao C, Zhan S, et al. (2020). Relationship between triglyceride glucose index and the incidence of non-alcoholic fatty liver disease in the elderly: a retrospective cohort study in China. BMJ Open. 10(11):e039804.
- Guo W, Lu J, Qin P, Li X, Zhu W, Wu J, et al. (2020). The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: a cross-sectional study in Chinese adults. Lipids Health Dis. 19(1).
- Kim HS, Cho YK, Kim EH, Lee MJ, Jung CH, Park J-Y, et al. (2021). Triglyceride glucose-waist circumference is superior to the homeostasis model assessment of insulin resistance in identifying nonalcoholic fatty liver disease in healthy subjects. J Clin Med. 11(1):41.
- Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. (2021). Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 19(3):167–173.
- Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C et al. (2020). MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterol. 158(7).
- Eslam M, Newsome P N, Sarin S K, Anstee Q M, Targher G, Romero-Gomez M et al. (2020). A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 73(1):202–209.
- EASL–EASD–EASO. (2016). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 64(6):1388–1402.
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 67(1):328–357.
- Bedossa P. Pathology of non?alcoholic fatty liver disease. Liver Int. 2017;37(S1):85–9. http://dx.doi.org/10.1111/liv.13301.
- Tagkou NM, Goossens N. (2023). Nichtalkoholische Fettleber – Diagnose und Therapie in 2022. Schweiz Gastroenterol. 4(1):27–37.
- Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. (2020). Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 17(4):e1003100.
- Engin A. (2017). The definition and prevalence of obesity and metabolic syndrome. In: Obesity and Lipotoxicity. Cham: Springer International Publishing; 1–17.
- Lee J-H, Jeon S, Lee HS, Kwon Y-J. (2023). Association between waist circumference trajectories and incident non-alcoholic fatty liver disease. Obes Res Clin Pract. 17(5):398–404.
 Abstract
Abstract  PDF
PDF